
 |
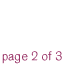 |
|||||||
  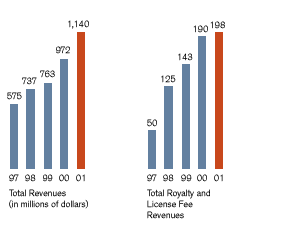 |
In 2001, we exceeded our stated financial objectives for the third consecutive year. Total revenues rose 17 percent, to $1.1 billion, compared to $972 million in 2000. This achievement reflects revenue growth in all three of our businesses, continued improvements in our operating efficiencies, and steady increases in our royalty income. During the year, we also continued to expand the markets for key products, advanced multiple candidates in our product pipeline, and strengthened our management team. At the same time, we renewed our strategic focus, restructured our research and development organization, and enhanced our commercial capabilities, all with the goal of positioning Chiron for improved productivity and continued success. BioPharmaceuticals: Maximizing Growth Opportunities Chiron’s BioPharmaceuticals business grew significantly in 2001, primarily due to the excellent performance of TOBI®, our inhaled antibiotic treatment for lung infections in cystic fibrosis patients. In the past year, we successfully launched TOBI into major European markets while increasing market penetration in the U.S., pointing to the strength of our global commercial infrastructure. Revenues from TOBI grew 43 percent to $123 million in 2001. To fully leverage the success of this program, we are developing new indications for TOBI, as well as a second-generation product in collaboration with Inhale Therapeutic Systems, Inc. U.S. patient demand for Proleukin®, our leading cancer product, increased by about six percent in 2001. Proleukin is a powerful immune system stimulator whose potential for improving patient outcomes in different types of cancer and HIV is being evaluated in numerous clinical trials. Worth noting are encouraging results from our Phase I study of Proleukin in combination with Rituxan® (rituximab) in non-Hodgkin’s lymphoma, suggesting a potential role for Proleukin in enhancing the effects of certain monoclonal anti-body-based treatments. We continued to see growth in sales of our multiple sclerosis drug, Betaseron®, a 15 percent increase over 2000. Chiron and its partner Schering AG are developing next generation products and have received FDA approval of an easier-to-use room-temperature formulation of Betaseron. Vaccines: Providing Novel Solutions Sales of both our novel and conventional vaccines were strong in 2001, underscoring our commitment to grow and develop this business. In the case of Menjugate™, our conjugate meningococcal C vaccine, total revenues have climbed to over $220 million since the product’s launch in 2000. Menjugate recently cleared the Mutual Recognition Procedure (MRP) in Europe. We also realized revenue growth from Menjugate’s use in late 2001 in a universal vaccination campaign in Quebec. Chiron is building on Menjugate’s success to develop new meningitis vaccines targeted against other types of meningococcal meningitis, including meningococcal B disease. Other strong performers among our more than 30 pediatric and adult vaccines include products to protect against flu, tick-borne encephalitis, rabies, polio, DTP (diphtheria, tetanus, pertussis combined vaccines), and MMR (measles, mumps, and rubella). Blood Testing: Innovating for Greater Blood Safety In 2001, we continued to see worldwide adoption for the Procleix™ System, an assay and instrument system that employs nucleic acid testing (NAT) technology to improve the safety of the world’s blood supply. The growing adoption of the Procleix HIV-1/HCV Assay signals widespread recognition of its clinical success in detecting the presence of HIV-1 or HCV during the very early stages of infection. With 50 million annual blood donations in North America, Europe, and Asia-Pacific, the potential market for NAT blood screening is $500 million or more. In the U.S., the Procleix system was approved by the FDA in February 2002. This event, accompanied by continued expansion into new markets, should fuel substantial revenue growth in the coming year. To support this growth and respond to market needs, we have enhanced our commercial capabilities and are developing additional assays with our partner, Gen-Probe Incorporated. |
|||||||